- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
Background
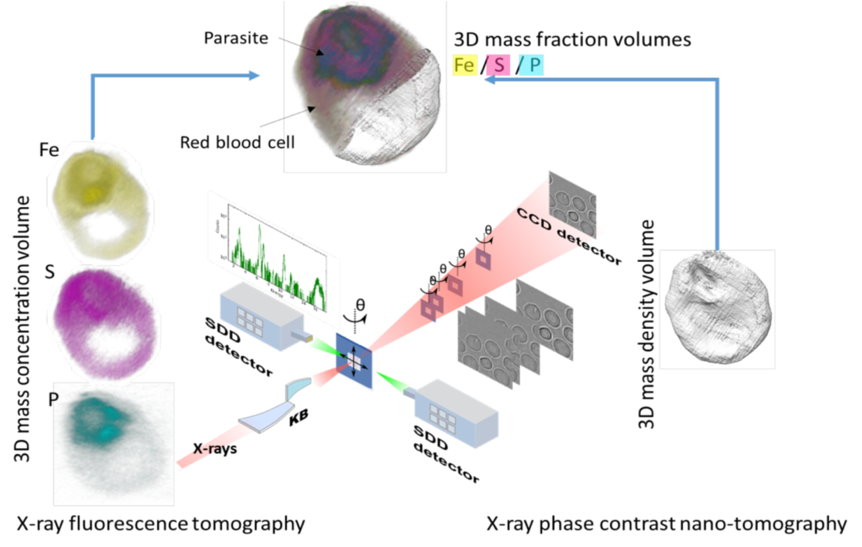
‘‘New Truths become evident when new tools become available’’, a famous quote from Rosalyn Sussman Yalow (Nobel Prize in Physiology and Medicine, 1977), is exemplified today by the impressive number of techniques which have provided breakthroughs in cellular machinery, such as far-field fluorescence nanoscopy or cryo-electron tomography. Extending these techniques to the X-ray domain offers unique opportunities to probe sub-cellular chemical processes. For example, soft X-ray microscopes have established capabilities in absorption contrast imaging of thick hydrated biological material in their near-native environments at spatial resolutions approaching 30 nm, well beyond those achievable with conventional visible light microscopy [1,2]. In the past decade, there has been a strong tendency in X-ray microscopy to develop alternative contrast mechanisms and spectroscopic methods, which can provide both valuable complementary information on the sample nature and/or a reduction of the necessary radiation doses [3,4]. Simultaneously, the development of high brilliance, high energy synchrotrons, coupled with advances in manufacturing technologies of focusing optics, has led to significant improvements in sub-micrometer probes for spectroscopy, diffraction and imaging applications in the hard X-rays regime (> 1 keV) and very recently opening the way to 2D/3D cryo Hard X-ray cryo-nanoprobe ID16A lead by P. Cloetens at ESRF (Figure below).
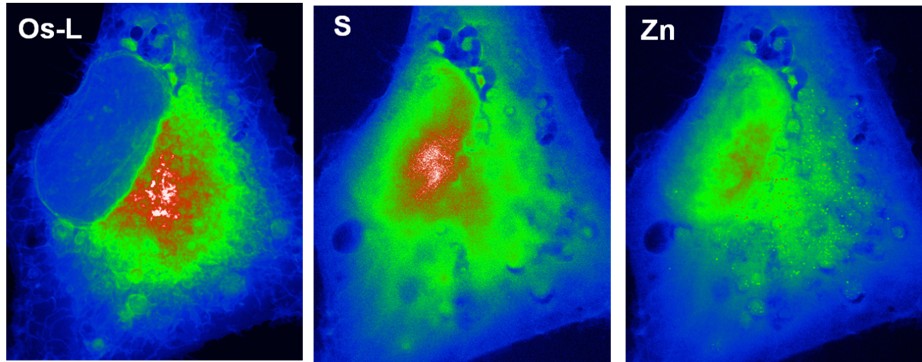
Living systems, for survival, depending on their ability to accumulate, release and use certain elements, particularly metal ions, to define a certain composition that is held constant within a given homeostatic state. Several essential metal ions participate in the control of numerous metabolic and signaling pathways, but their rich coordination chemistry and redox properties confer them a propensity to randomly coordinate and catalytically react inside the cell with protein sites other than those tailored for that purpose. Indeed, about one-third of all structurally characterized proteins are metalloproteins and bound metal ions or co-factors, which play a pivotal role in the structure-function relationship of proteins and other bio-molecules. In addition, all these cellular essential metals are also potentially toxic. Thus, a number of sophisticated networks of trafficking pathways are available to tightly regulate their uptake, intracellular transport, and compartmentalization, and to avoid their toxic side effects. However, in spite of all the progress made, we are still merely on the brink of understanding these processes.
The synchrotron micro- and nano-probe spectroscopic techniques in continuous development and progress allow a unique mesoscale capability to explore and elucidate the distribution, concentration and chemical state of metals inside tissues and cells at the organelle level. This contribution is not only highly challenging but represents important objectives in modern analytical chemistry and an essential step towards the precise understanding of some cellular physiopathological or toxicological processes. We developed expertise in sub-cellular imaging for a better understanding of the metallobiology of biologic systems and we actively participated in the development of the synchrotron nanochemical imaging and particularly in cryo-capabilities.
References
- C. Jacobsen, "Soft x-ray microscopy," Trends in Cell Biology, vol. 9, pp. 44-47, 1999.
- G. Schneider, P. Guttmann, S. Heim, S. Rehbein, F. Mueller, K. Nagashima, et al., "Three-dimensional cellular ultrastructure resolved by X-ray microscopy," Nature methods, vol. 7, p. 985, 2010.
- M. D. de Jonge and S. Vogt, "Hard X-ray fluorescence tomography—an emerging tool for structural visualization," Current opinion in structural biology, vol. 20, pp. 606-614, 2010.
- E. Lombi and J. Susini, "Synchrotron-based techniques for plant and soil science: opportunities, challenges and future perspectives," Plant and Soil, vol. 320, pp. 1-35, 2009.
- Y. Yang, F. Fus, A. Pacureanu, J. C. da Silva, W. De Nolf, C. Biot, S. Bohic, and P. Cloetens, "Three-Dimensional Correlative Imaging of a Malaria-Infected Cell with a Hard X-ray Nanoprobe" Analytical Chemistry 2019 91 (10), 6549-6554.
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn