- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
Ventilation inhomogeneity in lung disease
The uneven distribution of ventilation has major fundamental and clinical implications. Ventilation heterogeneity affects the matching of regional ventilation and perfusion leading to less efficient gas exchange, and can significantly affect the apparent degree of mechanical obstruction. Moreover, ventilation distribution importantly determines the distribution of inhaled medications and inhaled environmental pollutants.
K-edge subtraction imaging of regional lung structure and function takes full advantage of the high flux and energy-tunability of synchrotron radiation, and is applied to the pathophysiological investigation of lung diseases such as asthma and ventilator-induced lung injury as well as characterization of regional tissue perfusion in brain and liver.
One of the lung diseases where ventilation distribution inhomogeneity is enhanced is asthma. Asthma is characterized by the exaggerated contractile response of airways to various stimuli. For asthma diagnosis, airway responsiveness is often investigated using pharmacological substances that directly stimulate the airway smooth muscle. In asthmatic individuals however, airways constriction is indirectly induced by stimulation of inflammatory cells such as mast cells and eosinophils that release constricting mediators. Using KES imaging, it was demonstrated in anesthetized rabbits that when airway constriction is induced by the inhalation of histamine mimicking an asthma attack, regional ventilation becomes very heterogeneous with development of patchy areas of reduced ventilation (1-3). It was shown that the kinetics of airway response to histamine was very different in central versus small peripheral airways, with faster constriction and recovery in the lung periphery, while central airways were slower to reach their maximal constriction (1), occasionally showing paradoxical dilatations (2). This phenomenon may be due to the dynamic tidal expansion of proximal bronchi when distal airways constrict downstream, or serial airway interdependence (4).
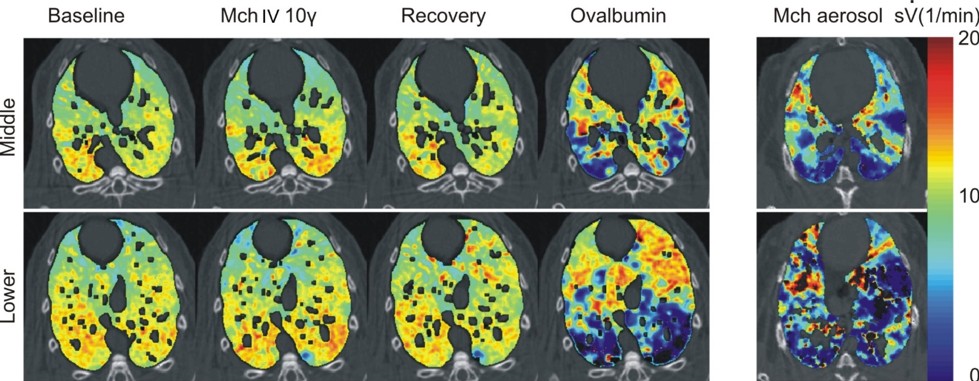
The mechanism through which airway smooth muscle constriction is induced results in radically different patterns of airways constriction (5). In rabbits sensitized to ovalbumin, airway constriction was induced either non-specifically through a pharmacological agent such as methacholine, which acts directly on the airway smooth muscle, or specifically through an allergen such as ovalbumin following allergic sensitisation, which constricts the airways indirectly by triggering the activation of mast cells and eosinophils. While methacholine induced mainly central airway constriction, the allergen caused a much more heterogeneous pattern of ventilation distribution in the lung periphery through constriction of more peripheral airways (Figure 1). Responses in airway resistance and tissue elastance measured by the forced oscillation technique were for the first time, demonstrated to be related to the central and peripheral airway responses, respectively (5). Since both constricting agents were injected in the blood, the more inhomogeneous airway reaction in allergic animals may have been due to the local heterogeneity of the distribution of the immune cells involved in the allergic reaction itself. This was investigated using KES imaging in ovalbumin-sensitized Brown-Norway rats, a species genetically prone to develop allergic sensitization (6). When the animals were exposed to allergen, and subsequently imaged while breathing a Xe and O2 gas mixture, patchy regions of ventilation defect developed in the lung periphery. Histologic examination of matching slices of the lung tissue revealed that total cellular, eosinophil and mast cell infiltrations were stronger within rather than outside the lung regions with reduced ventilation. Moreover, the inhomogeneous distribution of an inhaled aerosol containing environmental antigens or constricting agonists can result in an inhomogeneous peripheral airway response.
Acute Respiratory Distress Syndrome (ARDS), a condition characterized by heterogeneous inflammatory lesions of the lung blood-air barrier that result in leakage of protein-rich oedema fluid in the alveoli, with subsequent widespread alveolar flooding, closures and atelectasis (7). We have previously demonstrated how parameters such as positive end-expiratory pressure (PEEP) and affect regional lung function (Figure 2).
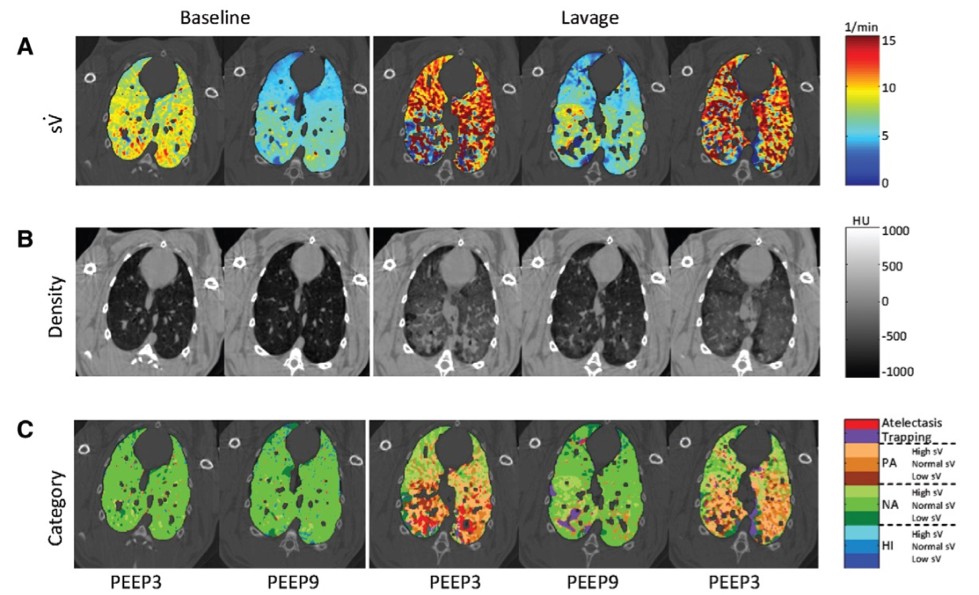
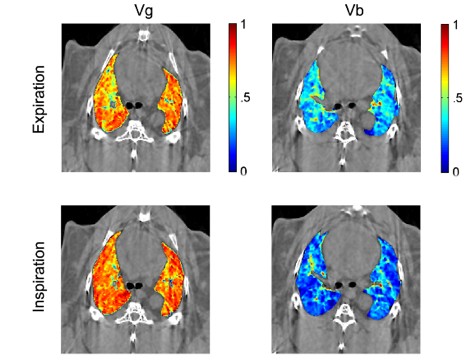
Mechanical ventilation, a crucial means of life support during acute respiratory failure or routine anaesthesia, can cyclically affect the regional distribution of blood volume in normal lung. Despite the importance of dynamic changes in the regional distributions of gas and blood during the breathing cycle for lung function in the mechanically ventilated patient, until recently no quantitative data on such cyclic changes were available. We recently introduced a modification of the K-edge subtraction CT (KES) that allows to quantitatively analyse regional lung ventilation, blood volume, and tissue structure at sub-acinar spatial resolution (9-11) within the breathing cycle (Figure 3). Using this technique, we demonstrated for the first time, cyclic variations in regional pulmonary blood and gas volume distributions during a single respiratory cycle.
Direct evidence of small airway closure in ARDS studied by propagation-based phase-contrast CT
Airway closure is thought to play an important role in acute respiratory distress syndrome (ARDS). Airway closure has been imaged for the first time in an ARDS model by synchrotron phase contrast imaging providing direct evidence of this phenomenon.
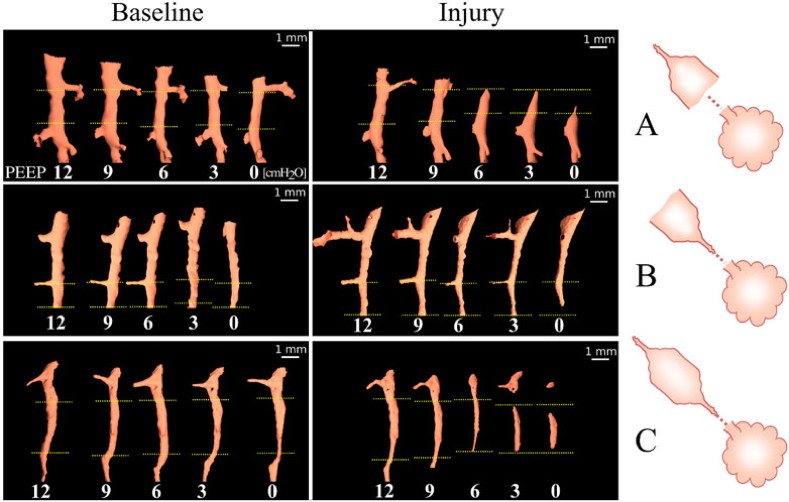
ARDS is an acute inflammatory lung condition associated with high permeability oedema, surfactant dysfunction and widespread collapse of pulmonary alveoli, called atelectasis, which leads to decreased lung compliance and volume (13). Clinicians have long suspected that the collapsibility of small airways is increased in this clinical syndrome, causing atelectasis (14, 15). While patients invariably require mechanical ventilation to survive, this life support measure can worsen lung injury due to exaggerated stress and strain applied to the tissue, which is magnified by mechanical inhomogeneity of lung tissue and atelectasis. Efforts to develop ventilation strategies that protect the lung, critically depend on our understanding of the mechanical behaviour of lung tissue and airways at the microscale. However, traditional computed tomography studies have not been able to clearly identify airway closure as a cause of atelectasis, due to their limited spatial resolution. To better identify the mechanisms involved in airway closure, it is necessary to use approaches that allow the study of individual airways. Here, the same individual small airways in intact lungs of anesthetised and mechanically ventilated rabbits with ARDS was studied using high-resolution synchrotron phase-contrast computed tomography at beamline ID17.
Propagation-based phase-contrast computed tomography, CT imaging was performed with a 47.5 μm3 voxel size, at positive end-expiratory pressures (PEEP) of 12, 9, 6, 3 and 0 cm H2O. The imaging sequence was repeated after lung injury induced by surfactant depletion through whole lung lavage and high-tidal volume mechanical ventilation in anesthetised rabbits. Cross-sections of the same individual airways were measured (Figure 4). Airway collapsibility increased in the injured lung with a significantly faster drop in airway cross-section as airway pressure decreased in small airways ranging between 210 – 1690 µm in radius at 12 cmH2O.
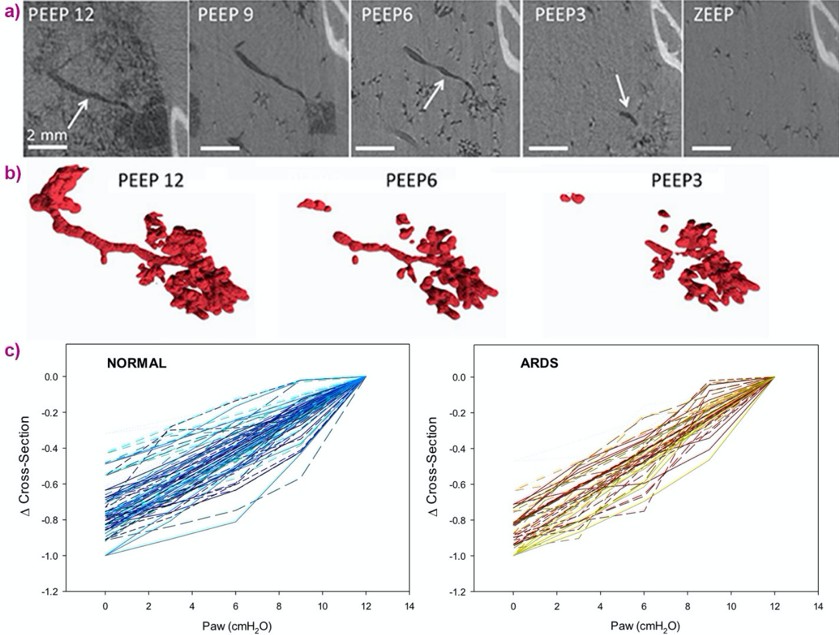
The main mechanism of airway closure in initially patent airways in ARDS was identified as “compliant collapse”. An example is shown in Figure 1a. Theoretical studies suggest that this mechanism involves an instability in the thickness of the fluid lining layer of the airway wall. Where this fluid layer thickens, the compressive forces that are generated cause a buckling of the airway wall that can lead to the collapse of the airway lumen along a certain length. Moreover, the analysis of airway cross-sections vs. length revealed that closure can occur at more than one site, sometimes leading to gas trapping and distension of the lumen (Figure 5).
Evidence of compliant collapse of airways in ARDS is indicated by the involvement of fluid layer movements at the microscopic level. This suggests that airway closure and reopening is not merely dependent on critical opening and closing pressures but is also a highly dynamic, time-dependent phenomenon (16). This may have implications for patients with ARDS under mechanical ventilation. Strategies aiming at reducing the time during which respiratory pressure is lowered during the breathing cycle may help prevent airway closure.
This study also underscores the need for further development of fast, high-resolution imaging techniques to dynamically image lung tissue mechanics at the microscale (17), ideally within the time span of a breath. The crucial link between microscopic lung tissue strain, and initiation and maintenance of inflammation, which leads to high mortality levels in ARDS patients, is still to be investigated, and dynamic X-ray microscopy with synchrotron sources promises to pave the way.
Quantitative Imaging of Regional Aerosol Deposition, Lung Ventilation and Morphology
The lung presents a large surface area in contact with the ambient atmosphere, and is the first barrier against environmentally inhaled particles, including atmospheric particulate matter and aerosols. Organic aerosols can carry antigenic compounds such as pollens, microorganisms and microbial toxins as well as environmental pollutants. On the other hand, inhaled therapy is the mainstay of treatment in obstructive lung diseases such as asthma, COPD and cystic fibrosis. This route of treatment has the advantage of minimizing systemic versus pulmonary effects, while having a rapid onset of action. Moreover, aerosol inhalation is increasingly being assessed as a route to administer other therapeutic agents such as viral vectors for gene therapy; peptides for the treatment of lung diseases such as vasoactive intestinal peptide to treat pulmonary hypertension; glutathione to treat cystic fibrosis; granulocyte-macrophage colony-stimulating factor to treat pulmonary alveolar proteinosis; calcitonin for postmenopausal osteoporosis; or insulin to treat diabetes.
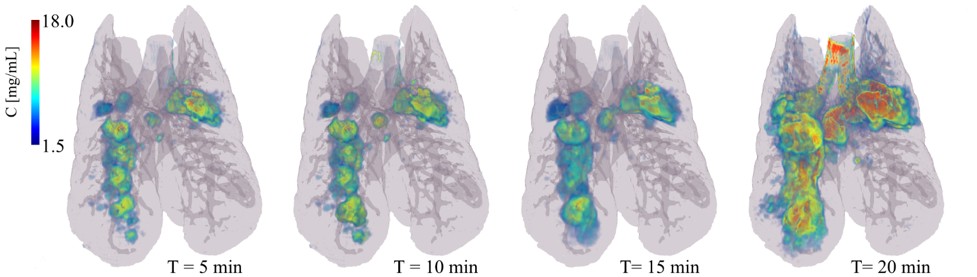
Aerosol particle deposition in the lower respiratory tract depends on a number of factors, including mass median aerodynamic diameter (MMAD), the inspiratory flow velocity and the flow regime, and the lung airway morphology. In the first few generations of the bronchial tree where the airflow velocity is relatively higher, aerosol particle deposition occurs mainly through inertial impaction, which is due to the inability of particles to follow rapid changes in gas flow direction. In this regard, airway narrowing due to bronchoconstriction, increased mucus secretion or remodelling as seen in obstructive respiratory diseases is a key determinant of the pattern of aerosol deposition in pathologic conditions, since the local gas flow velocity and likelihood of turbulent flow both increase in narrowed airways. Airway narrowing, can be very unevenly distributed among airway generations and can thereby contribute to the heterogeneity of aerosol deposition. This may in turn cause substantial differences in the regional delivery of medications to the lung. Currently, different strategies are being developed in order to target aerosol particles to specific regions of the lung, preferentially those that are more affected by a disease process. The success of such strategies in translating into clinical applications critically depends on imaging techniques allowing for both the quantitative determination of aerosol deposition, and the morphology of the bronchial tree, ideally using a single imaging modality with a high spatial resolution in order to also be applicable in preclinical small animal models. Traditional methods have mainly used radionuclide imaging of radioactively labelled aerosols, such as Single Photon Emission Computed Tomography (SPECT) or Positron Emission Tomography (PET) which are limited by their spatial resolution. Also, because these techniques do not allow imaging lung morphology, they are combined with computed x-ray tomography (CT), which requires co-registration of the acquired images on the combined instruments.
A novel application of synchrotron radiation K-edge subtraction CT imaging, is the quantitative mapping of lung aerosol particle deposition; lung morphology and regional lung ventilation. The latter is important for understanding aerosol deposition and its biological effects particularly in conditions of spatially inhomogeneous lung function, as is the case in lung diseases. Acquiring these data together has the potential of bringing new insight into the distribution of aerosol particle deposition. The imaging technique uses a single quantitative modality, with a comparatively high spatial as well as temporal resolution, allowing for repeated image acquisitions in in vivo preclinical models.
References
- Bayat S, Porra L, Suhonen H, Nemoz C, Suortti P, Sovijarvi AR. Differences in the time course of proximal and distal airway response to inhaled histamine studied by synchrotron radiation CT. J Appl Physiol (1985) 2006; 100: 1964-1973.
- Bayat S, Porra L, Suhonen H, Suortti P, Sovijarvi AR. Paradoxical conducting airway responses and heterogeneous regional ventilation after histamine inhalation in rabbit studied by synchrotron radiation CT. J Appl Physiol (1985) 2009; 106: 1949-1958.
- Porra L, Petak F, Strengell S, Neitola K, Janosi TZ, Suhonen H, Suortti P, Sovijarvi AR, Habre W, Bayat S. Acute cigarette smoke inhalation blunts lung responsiveness to methacholine and allergen in rabbit: differentiation of central and peripheral effects. Am J Physiol Lung Cell Mol Physiol 2010; 299: L242-251.
- Winkler T, Venegas JG. Complex airway behavior and paradoxical responses to bronchoprovocation. J Appl Physiol (1985) 2007; 103: 655-663.
- Bayat S, Strengell S, Porra L, Janosi TZ, Petak F, Suhonen H, Suortti P, Hantos Z, Sovijarvi AR, Habre W. Methacholine and ovalbumin challenges assessed by forced oscillations and synchrotron lung imaging. Am J Respir Crit Care Med 2009; 180: 296-303.
- Layachi S, Porra L, Albu G, Trouillet N, Suhonen H, Petak F, Sevestre H, Suortti P, Sovijarvi A, Habre W, Bayat S. Role of cellular effectors in the emergence of ventilation defects during allergic bronchoconstriction. J Appl Physiol (1985) 2013; 115: 1057-1064.
- Ware LB, Matthay MA. The acute respiratory distress syndrome. The New England journal of medicine 2000; 342: 1334-1349.
- Bayat S, Porra L, Albu G, Suhonen H, Strengell S, Suortti P, Sovijarvi A, Petak F, Habre W. Effect of positive end-expiratory pressure on regional ventilation distribution during mechanical ventilation after surfactant depletion. Anesthesiology 2013; 119: 89-100.
- Porra L, Suhonen H, Suortti P, Sovijarvi AR, Bayat S. Effect of positive end-expiratory pressure on regional ventilation distribution during bronchoconstriction in rabbit studied by synchrotron radiation imaging. Critical care medicine 2011; In press.
- Suhonen H, Porra L, Bayat S, Sovijarvi AR, Suortti P. Simultaneous in vivo synchrotron radiation computed tomography of regional ventilation and blood volume in rabbit lung using combined K-edge and temporal subtraction. Phys Med Biol 2008; 53: 775-791.
- Porra L, Monfraix S, Berruyer G, Le Duc G, Nemoz C, Thomlinson W, Suortti P, Sovijarvi AR, Bayat S. Effect of tidal volume on distribution of ventilation assessed by synchrotron radiation CT in rabbit. Journal of applied physiology 2004; 96: 1899-1908.
- Porra L, Broche L, Degrugilliers L, Albu G, Malaspinas I, Doras C, Wallin M, Hallback M, Habre W, Bayat S. Synchrotron Imaging Shows Effect of Ventilator Settings on Intra-breath Cyclic Changes in Pulmonary Blood Volume. Am J Respir Cell Mol Biol 2017.
- Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol 2005; 33: 319-327.
- Hedenstierna G, McCarthy GS. Airway closure and closing pressure during mechanical ventilation. Acta Anaesthesiol Scand 1980; 24: 299-304.
- Baumgardner JE. (Airway) Closure, at Last! Crit Care Med 2019; 47: 1281-1282.
- Broche L, Perchiazzi G, Porra L, Tannoia A, Pellegrini M, Derosa S, Sindaco A, Batista Borges J, Degrugilliers L, Larsson A, Hedenstierna G, Wexler AS, Bravin A, Verbanck S, Smith BJ, Bates JH, Bayat S. Dynamic Mechanical Interactions Between Neighboring Airspaces Determine Cyclic Opening and Closure in Injured Lung. Crit Care Med 2017; 45: 687-694.
- Fardin L, Broche L, Lovric G, Larsson A, Bravin A, Bayat S. Mapping cardiac-induced lung motion using high-resolution time-resolved phase-contrast synchrotron computed tomography. Eur Respiratory Soc; 2018.
Publications
Bayat et al. Functional lung imaging with synchrotron radiation: Methods and preclinical applications. Physica Medica. 2020
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn