- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
Here the energy-tunability and brilliance of synchrotron radiation has been instrumental in characterizing the biodistribution and uptake enhancement techniques within solid tumours. Ongoing studies in small animal models allow to better characterize and improve such approaches with the long-term goal of clinical translation. Our team has previously designed and completed the very first human phase I feasibility and safety study in human patients presenting brain metastases, completed in June 2017.
Background
Glioblastoma multiforme (GBM) is one of the most common central nervous system tumours. Despite aggressive treatment, which usually involves surgery, adjuvant chemotherapy with temozolomide and radiation therapy, GBM still has a poor prognosis: the median survival is ≈ 14.6 months [1]. Many studies aim to overcome several determinants of resistance to conventional treatments by using various approaches to improve the poor prognosis of GBM. These tumours are very resistant to chemotherapy and standard radiotherapy does not offer a solution because the dose required to sterilize tumour cells also causes necrosis of healthy brain tissue. Radiotherapy is based on energy deposition by secondary electrons after physical interactions between photons and tissues. Since the probability of photoelectric interactions increases considerably with the atomic number of the medium, the perfusion of the tumour with a molecule of high atomic number induces a dose increase delivered to the tumour while preserving the surrounding healthy tissues. Since the work of Hainfeld in 2004 [2], which has shown the effectiveness of gold nanoparticles under X irradiation to increase the survival of mice bearing mammary tumours, the scientific community has been very interested in this new therapeutic approach. The specific accumulation of nanoparticles in the tumour lesion can be achieved "passively" using partial disruption of the blood-brain barrier in brain tumours. Another phenomenon of retention (EPR effect) is also often observed with nanoparticles [3]. This results in a high concentration of nanoparticles in the tumour, compared to that seen in healthy tissues. We obtained promising results on models of glioma implanted in the rat (model F98), by combining the injection of absorbing elements (chemotherapy agents, contrast agents or nanoparticles) followed by a low energy X irradiation (80 keV) performed at the Grenoble synchrotron [4-12]. Various avenues of research have been explored. We hypothesized that a better distribution of drugs at the macroscopic and microscopic level would improve the efficiency of the treatment and that a fine modeling of the effects generated at the cellular level would make it possible to optimize the treatment conditions (choice of elements, radiation energy etc.). We also hypothesized that the use of nanoparticles containing elements of high atomic number (Gd, Au, Fe, Bi) would increase the efficiency of synchrotron radiotherapy. We modelled X-ray matter interactions at the cellular level in the presence of heavy elements in order to accurately calculate the dose distribution delivered to patients, at the macroscopic scale but also at the microscopic scale, around the nanoparticles.
Objectives
Optimization of the distribution of chemotherapy agents and nanoparticles
The optimization of the intracerebral bio-distribution of drugs remains a major problem that we face in the particular context of brain tumours. Indeed, the brain and a large part of the tumour are separated from the blood compartment by the blood-brain barrier (BBB). Drugs that pass through this barrier with difficulty, cannot homogeneously reach tumour cells and infiltrating cells located at a distance from the tumour. An alternative to getting around the BBB is to inject the drug locally. The product, which does not pass the BBB, will remain in the intracerebral compartment. We have implemented this technique to administer chemotherapy drugs (cisplatin, carboplatin) or nanoparticles of gold or gadolinium, in combination with radiotherapy [3-6]. Imaging is an essential tool for monitoring the bio-distribution of drugs in real time and ensuring that the dose-enhancer covers the targeted tumour area. Synchrotron imaging allows absolute quantification of the concentrations of the injected elements and thus accurately calculates the dose that is directly related to the concentration [7].
Preclinical studies
Preclinical studies have been conducted on models of rodent-implanted tumours (F98). These experiments were conducted in accordance with the recommendations of the Ministry of Agriculture and submitted to ethics committees for advice. The ESRF has an approved pet shop and the team members involved in the preclinical trials have an authorization to experiment.
Radiation therapy in the presence of nanoparticles
The interest of using nanoparticles for this approach is based on several points: Nanoparticles behave as small secondary sources of electrons, capable of causing significant cellular damage, the dose in the immediate vicinity of nanoparticles is very high (several tens of Gy). Some nanoparticles are also contrast agents for MRI or X-ray imaging (theranostic agent). On the other hand, it is possible to functionalize them to increase tumour targeting. The results obtained in the context of the thesis of L Bobyk and F Taupin show that the presence of gold nanoparticles or gadolinium radio-sensitize very effectively tumour cells in vitro (dose enhancement factor of 2 with 10 mg / ml gold) [1-4]. A preclinical test was conducted on rodents carrying F98 glioma after intracerebral injection of gold nanoparticles (AuNP). The results of this study published in the journal "nanomedicine" show a significant increase in the survival of the rats that received the combination of treatment (AuNP and synchrotron radiotherapy) in comparison with rats treated only with synchrotron radiotherapy [5]. Depending on the location of the nanoparticles at the sub-cellular level, the effectiveness of the treatment varies. It is therefore crucial to study finely the distribution of nanoparticles at different scales. On a macroscopic scale, bio distribution studies (synchrotron tomographic imaging) show that gold nanoparticles remain in high concentration (> 5 mg/ml) in the tumour zone for more than 24 hours after direct intracerebral injection.
At the sub-cellular level, in collaboration with Sylvain Bohic (member of our team), we have carried out studies to characterize by micro-fluorescence X synchrotron the distribution of nanoparticles (iron nanoparticles, and Gd contrast agents).
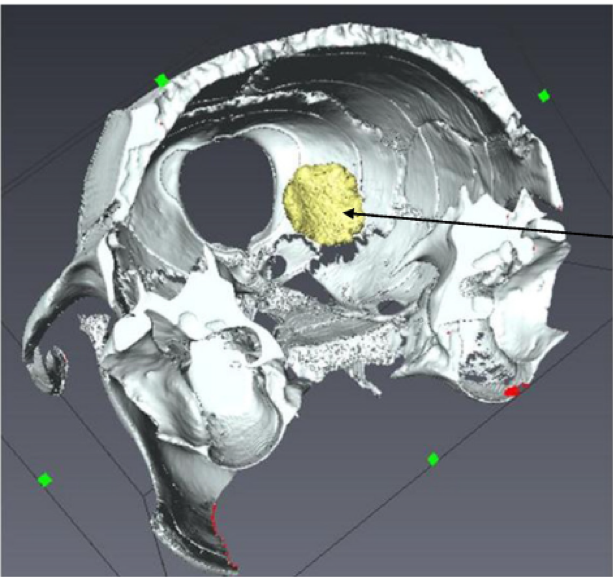
On the basis of these imaging results, we evaluated the dose distribution at the cellular and sub-cellular level using Monte Carlo methods [1]. The nanoparticles of Gd significantly increase the dose X received by the tumour cells, in particular at the energies of synchrotron radiation (50-80 keV). At high energy, a biological effect is also observed then that is not predicted modeling. A biological effect at the level of the cell cycle is probably responsible for this effect [2, 3]. The Gd nanoparticles have also been used in a clinical trial conducted at Grenoble CHU by Prof. J. Balosso and Dr. C Verry [4, 5].
We established a collaboration with a Korean team led by Dr. Jong Ki Kim (Catholic University of Daegu, South Korea), they had obtained at low energies very interesting survival results on a tumour model implanted in the mouse after intravenous injection of iron nanoparticles [1]. In 2017, we obtained funding through a Campus France "STAR" call for projects from the Ministry of Europe and Foreign Affairs to finance exchanges and workshops for two years.
Preclinical studies using iron oxide nanoparticles
The therapeutic value of iron nanoparticles for synchrotron radiotherapy will be evaluated by preclinical tests always performed in close collaboration with JL Ravanat (CEA Grenoble) and our South Korean collaborators. The possibility of using these nanoparticles as a contrast agent in MRI imaging will also be evaluated (collaboration E. Barbier and V Stupar GIN Grenoble). Having joined the COST RADIOMAG network in 2017, new collaborations could emerge to also use other properties of these iron nanoparticles such as hyperthermia to increase their therapeutic efficacy [1, 2].
Nanoscintillators: deep tissue photodynamic therapy
Photodynamic therapy (PDT) is a light-based cancer therapy that consists in activating non-toxic photosensitizers using visible light to induce cytotoxic species and lead to tumour destruction. PDT is currently used in clinic to treat superficial tumours such as non-melanoma skin cancers, or endoscopically-accessible tumours including bladder or prostate cancers.
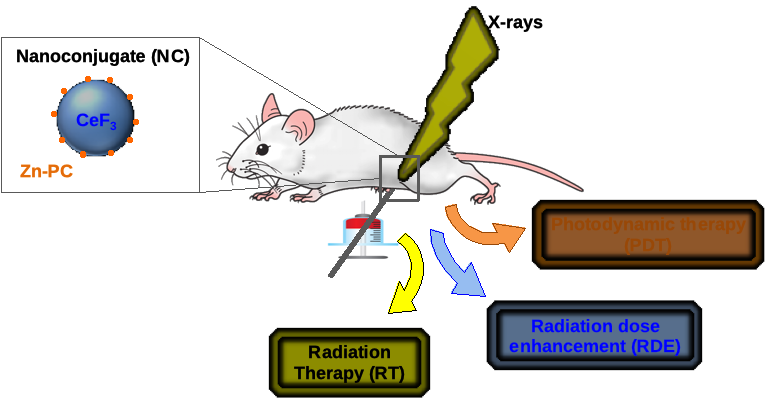
Despite its strong potential, PDT remains limited by the low penetration of light in tissues. In order to overcome this restriction, it has been proposed to conjugate the photosensitizers on to nanoscintillators. Those down-converting nanoparticles absorb X-ray and convert those penetrating radiations into visible light that can subsequently excite the photosensitizer and induce PDT independently of the tumour location. This concept has encountered a growing interest since its introduction in 2006 and we propose to investigate this radiation therapy/deep tissue PDT upon synchrotron radiation. In addition, as nanoscintillators are composed of high-Z atoms, the radiation dose enhancement effect will also be investigated. In order to verify these hypotheses and to study the underlying mechanisms, we propose a multidisciplinary project articulated in three axes.
First, we will develop a numerical simulation tool based on the Monte Carlo method that will be built on previous work. This tool will allow us to quantify the increase in dose deposition due to the accumulation of nanoscintillators in tumours as well as the maximum activation efficiency of the PDT effect as a function of parameters such as incident X-ray energy. and the characteristics of nanoscintillators (composition, size, concentration). The results of this first axis will guide the choice of nanomaterials to be synthesized as well as the conditions of excitations to use. The nanoconjugates will be synthesized by F. Chaput (ENS Lyon) and functionalized by Christelle Gateau (CEA Grenoble).
The second part of this project aims at characterizing the effectiveness of these nanomaterials in vitro on isolated DNA samples and on 3D culture of cancers in order to determine both the dose increase and the nature of the reactive species involved (collaboration JL Ravanat, CEA Grenoble).
Finally, the third aim of this project consists in studying in vivo the efficacy of the selected nanoscintillator / photosensitizer conjugates. The in vivo preclinical tests will be conducted on models of glioma and ovarian tumours implanted in rodents (rat and mice respectively). While the glioma-bearing rat models (F98) will be developed in the group, the ovarian cancer model implanted in mice will be developed in close collaboration with J.L. Coll's team from the Institute of Advanced Bioscience (L. Sancey), as well as a hospital expert in gynecologic oncology (Dr Michy, CHU Grenoble). The radiotherapy will be delivered via two sources of irradiation for which the results will be compared: conventional sources used clinically for radiotherapy (energy in the MeV range) and the synchrotron source (energy ranging between 30 and 100 keV) that promotes the expected dose enhancement effect.
Study of X-ray dose deposition in the presence of copper ions
The understanding of energy deposition at the cell level when X-rays interact with the cell in the presence of exogenous atom of medium or high atomic numbers remains incomplete, particularly when relating the physical X-ray dose deposited to the biological effects as a function of X-ray photon energy. We have worked on this topic since 2000. Starting in 2006 we focussed on the effect of kilovoltage X-rays on glioma cells in the presence of bismuth (Z=83), the choice of cell line being dictated by our team’s aim to develop more effective options in the treatment of glioblastoma and other cerebral tumours (the unpublished work can be found in part in the stage reports of Grishma Patel, Estelle Morcillo, Bastien Laperoussaz, Baptiste Laurent, David Clément, and the Laurea thesis of Giordano Traini). In this framework it appeared that bismuth could have a radioprotective effect, particularly following cell survival experiments with F98 cells pre-incubated with AguIX® nanoparticles containing an equal number of gadolinium and bismuth atoms. In 2016 we thus turned to the use of nanoparticles containing a high Z element (gadolinium, gold) coupled to a medium Z element, more specifically copper ions. The purpose of the copper here is to stimulate the formation of free radicals, and possibly increase the chemical toxicity through a change in valence of the copper ions induced by the secondary electrons produced following the irradiation of the heavy element. A cell survival experiment carried out end of 2016 using AguIX® nanoparticles with 70% gadolinium, 30% copper encourages us to pursue in this direction. This work is on hold since the previously used irradiator is now out of order but we hope that operation will resume soon.
Cell survival measurements have numerous sources of uncertainty. It is essential to carry them out under good experimental conditions: controlled conditions for cell culture, limited travel time, isothermic conditions, irradiation dosimetry, post-irradiation clonogenic assays carried out according to rigorous protocols, and strict limitations of dead-times. The availability of a high-performance kilovoltage irradiator in the Grenoble metropolitan area is therefore a necessary condition to the fruitful continuation of this work. Before being carried out with monochromatic synchrotron radiation, cell experiments need pre-testing with an irradiator.
References
- Durante, M. and J.S. Loeffler, Charged particles in radiation oncology. Nat Rev Clin Oncol, 2010. 7(1): p. 37-43.
- Bianco, J., et al., On glioblastoma and the search for a cure: where do we stand? Cell Mol Life Sci, 2017. 74(13): p. 2451-2466.
- Hainfeld, J.F., D.N. Slatkin, and H.M. Smilowitz, The use of gold nanoparticles to enhance radiotherapy in mice. Physics in Medicine and Biology, 2004. 49(18): p. N309-15.
- Adam, J.F., et al., Prolonged survival of Fischer rats bearing F98 glioma after iodine-enhanced synchrotron stereotactic radiotherapy. Int J Radiat Oncol Biol Phys, 2006. 64(2): p. 603-11.
- Delorme, R., et al., Comparison of gadolinium nanoparticles and molecular contrast agents for radiation therapy-enhancement. Med Phys, 2017. 44(11): p. 5949-5960.
- Taupin, F., et al., Gadolinium nanoparticles and contrast agent as radiation sensitizers. Physics in Medicine and Biology, 2015. 60(11): p. 4449-64.
- Bobyk, L., et al., Photoactivation of gold nanoparticles for glioma treatment. Nanomedicine, 2013. 9(7): p. 1089-97.
- Ceberg, C., et al., Photon activation therapy of RG2 glioma carrying Fischer rats using stable thallium and monochromatic synchrotron radiation. Physics in Medicine and Biology, 2012. 57(24): p. 8377-91.
- Bobyk, L., et al., Intracerebral delivery of carboplatin in combination with either 6 MV photons or monoenergetic synchrotron X-rays are equally efficacious for treatment of the F98 rat glioma. J Exp Clin Cancer Res, 2012. 31: p. 78.
- Edouard, M., et al., Treatment plans optimization for contrast-enhanced synchrotron stereotactic radiotherapy. Med Phys, 2010. 37(6): p. 2445-56.
- Rousseau, J., et al., Efficacy of intracerebral delivery of cisplatin in combination with photon irradiation for treatment of brain tumors. J Neurooncol, 2010. 98(3): p. 287-95.
- Rousseau, J., et al., Intracerebral delivery of 5-iodo-2'-deoxyuridine in combination with synchrotron stereotactic radiation for the therapy of the F98 glioma. J Synchrotron Radiat, 2009. 16(Pt 4): p. 573-81.
- Prezado, Y., et al., Biological equivalent dose studies for dose escalation in the stereotactic synchrotron radiation therapy clinical trials. Med Phys, 2009. 36(3): p. 725-33.
- Rousseau, J., et al., Efficacy of intracerebral delivery of Carboplatin in combination with photon irradiation for treatment of F98 glioma-bearing rats. Int J Radiat Oncol Biol Phys, 2009. 73(2): p. 530-6.
- Adam, J.F., et al., Heavy element enhanced synchrotron stereotactic radiotherapy as a promising brain tumour treatment. Phys Med, 2008. 24(2): p. 92-7.
- Rousseau, J., et al., Enhanced survival and cure of F98 glioma-bearing rats following intracerebral delivery of carboplatin in combination with photon irradiation. Clin Cancer Res, 2007. 13(17): p. 5195-201.
- Boudou, C., et al., Polymer gel dosimetry for synchrotron stereotactic radiotherapy and iodine dose-enhancement measurements. Physics in Medicine and Biology, 2007. 52(16): p. 4881-92.
- Rousseau, J., et al., Convection-enhanced delivery of an iodine tracer into rat brain for synchrotron stereotactic radiotherapy. Int J Radiat Oncol Biol Phys, 2007. 68(3): p. 943-51.
- Boudou, C., et al., Monte Carlo dosimetry for synchrotron stereotactic radiotherapy of brain tumours. Physics in Medicine and Biology, 2005. 50(20): p. 4841-51.
- Adam, J.F., et al., Enhanced delivery of iodine for synchrotron stereotactic radiotherapy by means of intracarotid injection and blood-brain barrier disruption: quantitative iodine biodistribution studies and associated dosimetry. Int J Radiat Oncol Biol Phys, 2005. 61(4): p. 1173-82.
- Boudou, C., et al., Synchrotron stereotactic radiotherapy: dosimetry by Fricke gel and Monte Carlo simulations. Physics in Medicine and Biology, 2004. 49(22): p. 5135-44.
- Prezado, Y., et al., Dosimetry protocol for the forthcoming clinical trials in synchrotron stereotactic radiation therapy (SSRT). Med Phys, 2011. 38(3): p. 1709-17.
- Barth, R.F., et al., Comparison of intracerebral delivery of carboplatin and photon irradiation with an optimized regimen for boron neutron capture therapy of the F98 rat glioma. Appl Radiat Isot, 2011. 69(12): p. 1813-6.
- Yang, W., et al., Convection enhanced delivery of carboplatin in combination with radiotherapy for the treatment of brain tumors. J Neurooncol, 2011. 101(3): p. 379-90.
- Verry, C., et al., MRI-guided clinical 6-MV radiosensitization of glioma using a unique gadolinium-based nanoparticles injection. Nanomedicine (Lond), 2016. 11(18): p. 2405-17.
- Sancey, L., et al., The use of theranostic gadolinium-based nanoprobes to improve radiotherapy efficacy. Br J Radiol, 2014. 87(1041): p. 20140134.
- Delorme, R., et al., Comparison of Gadolinium Nanoparticles and Molecular Contrast Agents for Radiation Therapy-Enhancement. Medical Physics, 2017.
- Choi, G.H., et al., Photon activated therapy (PAT) using monochromatic synchrotron X-rays and iron oxide nanoparticles in a mouse tumor model: feasibility study of PAT for the treatment of superficial malignancy. Radiat Oncol, 2012. 7: p. 184.
- Taupin, F., et al., Anti-canceral therapy by gold nanoparticle photoactivation. Bulletin Du Cancer, 2011. 98: p. S60-S61.
- Mallidi, S., et al., Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics, 2016. 6(13): p. 2458-2487.
- Obaid, G., et al., Photonanomedicine: a convergence of photodynamic therapy and nanotechnology. Nanoscale, 2016. 8(25): p. 12471-503.
- Bulin, A.L., et al., Modelling energy deposition in nanoscintillators to predict the efficiency of the X-ray-induced photodynamic effect. Nanoscale, 2015. 7(13): p. 5744-51.
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn